ByHeart Formula Recall: Infant Botulism Alert
Urgent Recall: ByHeart Infant Formula Linked to Botulism Outbreak
Parents and caregivers nationwide are urged to immediately stop using certain lots of ByHeart Whole Nutrition Infant Formula after federal health officials linked the product to a multistate infant botulism outbreak. The recall issued by ByHeart Inc. on November 8, 2025, comes amid 13 confirmed hospitalizations across 10 states, with no deaths reported to date.
What Is Infant Botulism?
Infant botulism is a rare but serious illness caused by Clostridium botulinum bacteria. When ingested, these bacterial spores can colonize an infant's intestines and produce a potent neurotoxin. This toxin attacks the nervous system, leading to potentially life-threatening paralysis. The disease typically begins with subtle signs:
- Constipation (often the first symptom)
- Poor feeding (difficulty sucking or swallowing)
- Weak or altered cry
- Loss of head control (floppy baby syndrome)
- Decreased facial expression
Without prompt treatment, symptoms can progress to muscle weakness, respiratory failure, and paralysis requiring intensive hospital care.
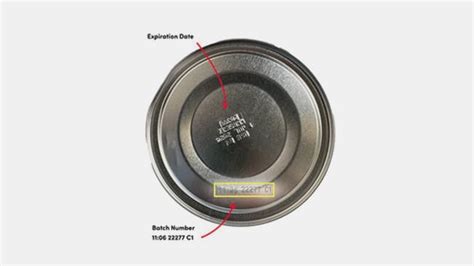
The Recall: Specific Products and Lot Numbers
The recall specifically targets two lots of ByHeart Whole Nutrition Infant Formula:
- Lot 206VABP/251261P2 (Use by: December 1, 2026)
- Lot 206VABP/251131P2 (Use by: December 1, 2026)

Critical Actions for Parents and Caregivers
"Do not use recalled infant formula. Throw it away or return it to where you bought it." - CDC
If you have either of the recalled lots: Stop use immediately. Record the lot number from the bottom of the packaging and:
- Discard the formula safely
- Return it to the point of purchase for a refund
- Thoroughly clean and sanitize bottles, nipples, and surfaces that may have contacted the formula using hot soapy water or a dishwasher
Important: Symptoms of infant botulism can take days to weeks to appear after consuming contaminated formula. Even if your baby appears healthy now, remain vigilant for at least 30 days after last use.
Seeking Immediate Medical Care
Contact a healthcare provider immediately if your infant has consumed the recalled formula and shows any of these warning signs:
- Lethargy or unusual drowsiness
- Difficulty swallowing or sucking
- Weak cry or diminished facial expression
- Loss of head control (inability to hold head up)
- Constipation lasting more than 3 days
Early treatment with BabyBIG® (botulism immune globulin) is critical and can significantly reduce complications. Healthcare providers should consult with the Infant Botulism Treatment and Prevention Program at 510-231-7600 for suspected cases.
The Investigation: What We Know
The Centers for Disease Control and Prevention (CDC), FDA, and state health departments are investigating 13 confirmed cases of infant botulism linked to ByHeart formula since August 2025. Affected states include Arizona, California, Illinois, Minnesota, New Jersey, Oregon, Pennsylvania, Rhode Island, Texas, and Washington.
While officials have not yet confirmed Clostridium botulinum in tested formula samples, California health authorities reported a significant increase in cases among babies fed ByHeart products. The FDA is analyzing remaining formula samples to identify the contamination source and determine if other products are affected.
ByHeart stated: "The FDA has not identified a direct link between any infant formula and these cases and there is no historical precedent of infant formula causing infant botulism." However, the company initiated a voluntary recall "to remove any potential risk."
What's Next?
Investigations continue as health officials:
- Trace the supply chain for contamination sources
- Monitor for new cases in the coming weeks
- Share findings with formula manufacturers to prevent future outbreaks
For updates, visit the CDC Botulism Outbreak Investigation Page or the FDA Recall Portal.
Protecting Your Baby: Key Takeaways
1. Check Your Formula: Verify lot numbers against the recall list immediately.
2. Discard Safely: Do not use or sell recalled products.
3. Monitor Symptoms: Watch for constipation, feeding difficulties, or floppiness for at least 30 days.
4. Seek Care Early: Contact providers at the first sign of symptoms.
5. Sanitize Thoroughly: Clean all feeding equipment and surfaces.
This recall underscores the critical importance of infant formula safety protocols. While ByHeart represents a small market share, no amount of risk is acceptable for our most vulnerable population. Stay informed, stay vigilant, and prioritize your baby's health above all.
Share this article
David Kim
Health and science reporter with a background in medicine. Passionate about making complex medical topics accessible.